All roads LFMS lead to the Harvard Low Field Magnetic Stimulation Lab. But please follow along with what led me here in the story below.
Harvard’s Low Field Magnetic Stimulation Lab
I came across Low Field Magnetic Stimulation, or LFMS, in a recent panel talk from SXSW: Superbugs, Magnets & More: Medicine’s Comeback Kids – SXSW Interactive 2015. The conversation is particularly interesting considering panelist Dr. Bennett Shapiro’s background. At Merck he led research that developed over 25 drugs and vaccines. (The panel touches on magnets, phage therapy and fecal transplants, for example.)
Dr. Shapiro’s company (he is the co-founder and non-executive director), Pure Tech Health created Tal Medical, to develop an LFMS treatment/device.
This is most likely the nexus for looking into LFMS in the first place:
Anecdotal reports have suggested mood improvement in patients with bipolar disorder immediately after they underwent an echo-planar magnetic resonance spectroscopic imaging (EP-MRSI)… Low-Field Magnetic Stimulation in Bipolar Depression Using an MRI-Based Stimulator (Found on the Tal Medical publications page.)
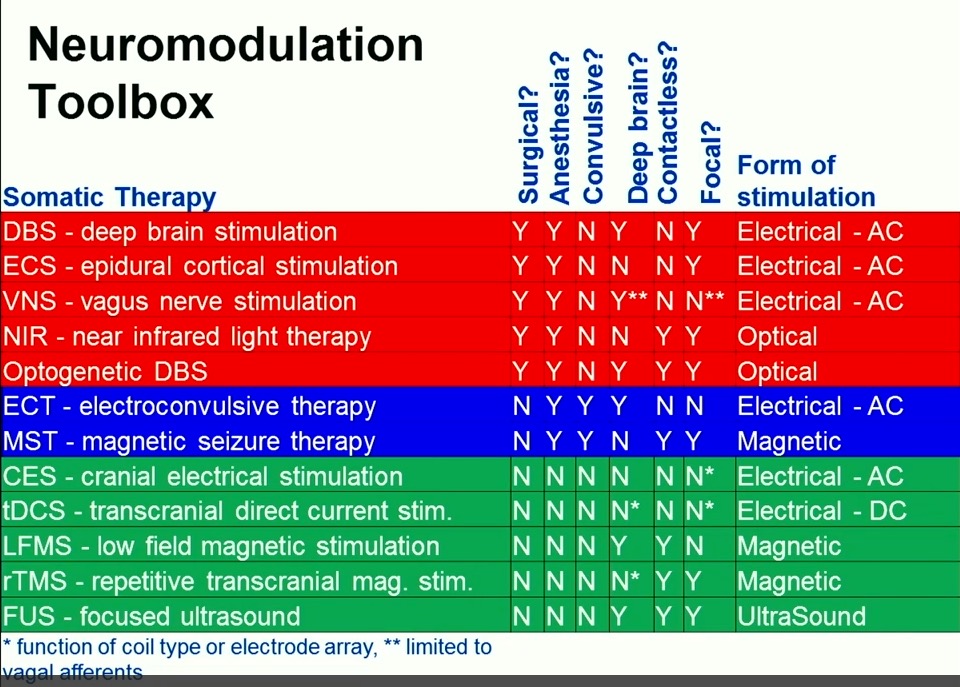
The panel discusses TMS, which has recently been approved by the FDA for treatment of depression. But Dr. Shapiro goes on to discuss LFMS, which (to my readers anyway) is especially interesting because it uses so little power to achieve its effects. (As opposed to TMS which is too complicated and powerful to ever become part of the DIY community. Never say never!)
In 2013, Tal received initial proof-of-concept data from a randomized, double-blind, sham-controlled trial in patients with major depressive and bipolar disorders conducted by McLean Hospital, a leading psychiatric research hospital affiliated with Harvard Medical School. In the study, a single 20-minute treatment demonstrated rapid onset of action, substantial effect size, and a strong safety profile. Given this unique, rapid effect of LFMS treatment, the National Institute of Mental Health has selected LFMS for a multi-site clinical trial. The trial is examining the efficacy and durability of the treatment over an extended period of time in patients with major depressive disorder.
Researching possible patents led to Michael Rohan, Ph.D. and the Harvard Low Field Magnetic Stimulation Lab. I assume there is a partnership between the Harvard Lab, McLean Hospital and Tal Medical, though I could not find any formal announcement. Click through the lab link to do a deeper dive into ongoing research they are presently involved with, including clinical trials.